KIT Blog
URGENT: Please pray ENLIST makes break-throughs in the treatment of leprosy reaction!
-
 Admin
Admin
- May 21, 2021
- Global,
- Understanding Neglected Tropical Diseases
By Dr Deanna Hagge
Senior Research Advisor
The Leprosy Mission
ENLIST is a group of specialists including doctors from The Leprosy Mission hospitals in India, Nepal, and Bangladesh, as well as experts from Brazil, Sri Lanka, Ethiopia, the Philippines, another Indian partner, and myself. These specialists have been brought together through Dr Steve Walker and Dr Diana Lockwood of the London School of Tropical Medicine and Hygiene to look at the issue of Type 2 Reaction (ENL).
It is very important for us to work as a global partnership because it’s almost impossible for any one site to perform a large, comprehensive study. Through ENLIST, we can design and conduct ENL studies that need to happen, involving expertise from around the globe.
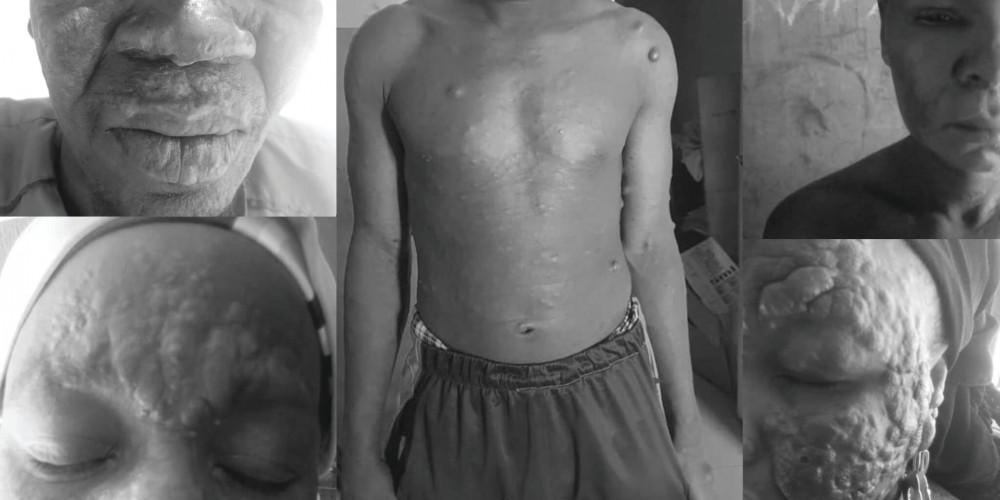
Through Leprosy Research Initiative funding, we’ve been able to develop an ENL Severity Scale to grade 10 major symptoms of ENL. This provides a global tool for measuring and comparing ENL patients to each other, understanding how symptoms change with treatment, and allowing us to classify a patient’s condition as mild or moderate-to-severe ENL.
This severity score is a really important starting place, because now we have a method of testing new ENL treatments and getting an accurate assessment of how well they are working.
We are now using this tool to conduct a trial which will see us combine Prednisolone with another drug called Methotrexate. The hope is that, by using the two together, we can slowly wean a patient off Prednisolone. This may be possible for some but perhaps not for very severe ENL cases.
We are also at the very early stages of looking at whether an entirely new drug (so new that it doesn’t yet have a name) could be used to treat ENL. The early results are hugely promising and suggest we may be able to treat ENL with perhaps only a month of drug treatment with no major side effects. That would be revolutionary! One of the patients in the trial felt so good after treatment, that he did not want to take time from work to come in for a follow-up appointment. While this was not a positive for the study, it reminded me that this is what we want for our ENL patients.
To receive short, effective treatment such that returning to the doctor’s office is truly unnecessary.
But it will take time for us to trial this drug at every level, generating data to pass through all the regulatory standards and checkpoints required for international drug approval. In all, it could be mid-way through the 2020s, or even later, before it is ready to be used clinically.
With every ENL patient that walks through the door, there is a definite urgency to see change happen; and there are real reasons for hope.
Please remember to pray for us as we go about our work and pray for the patients we are trying to help out of dire situations. Thank you for all your support and God bless you!